
Cobalt alloys are a group of cobalt-chromium ‘super-alloys’ consisting of complex carbides in an alloy matrix. Let’s take a look at everything you need to know about cobalt alloys.
Elemental Cobalt
Physical Properties. With an atomic number of 27, cobalt falls between iron and nickel on the periodic table. The density of cobalt is 8.8 g/cm3 similar to that of nickel. Its thermal expansion coefficient lies between those of iron and nickel. At temperatures below 417°C cobalt exhibits a hexagonal close-packed structure. Between 417°C and its melting point of 1493°C, cobalt has a face-centered cubic structure.
The elastic modulus of cobalt is about 210 GPa (30 x 106 psi) in tension and about 183 GPa (26.5 x 106 psi) in compression.
Cobalt-Base Alloys
The cobalt-base alloys may be generally described as wear resistant, corrosion resistant, and heat resistant (strong even at high temperatures). Many of the properties of the alloys arise from the crystallographic nature of cobalt (in particular its response to stress), the solid-solution-strengthening effects of chromium, tungsten, and molybdenum, the formation of metal carbides, and the corrosion resistance imparted by chromium. Generally the softer and tougher compositions are used for high-temperature applications such as gas-turbine vanes and buckets. The harder grades are used for resistance to wear.
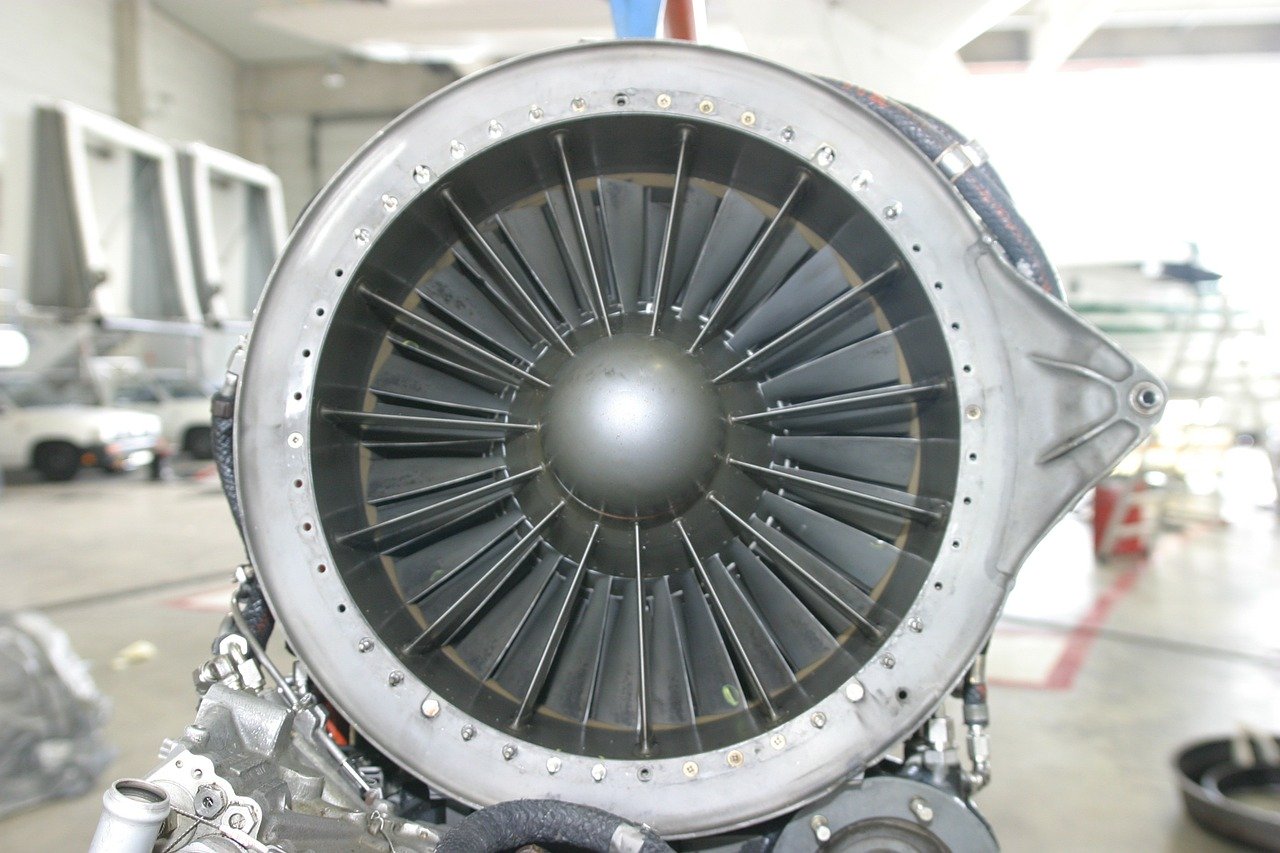
Cobalt-Base High-Temperature Alloys
For many years, the predominant user of high-temperature alloys was the gas turbine industry. In the case of aircraft gas turbines, the chief material requirements were elevated-temperature strength, resistance to thermal fatigue, and oxidation resistance. For land-base gas turbines, which typically burn lower grade fuels and operate at lower temperatures, sulfidation resistance was the major concern.
Today, the use of high-temperature alloys is more diversified, as more efficiency is sought from the burning of fossil fuels and waste, and as new chemical processing techniques are developed.
In general, cobalt-base high-temperature alloys have the following chemical composition:
Cr = 20-23%
W = 7-15%
Ni = 10-22%
Fe = 3% max
C = 0.1-0.6%
Co = rest of balance.
Manufacturing Process
Cobalt alloys are produced by a range of different processes or methods. These methods include wrought or hot forging, hard-faced deposit, powder metal, and finally, casting. It’s more difficult to machine and grind than steel. As a result, high-performance processing equipment and specialized machining tools are required. Typically, it is machined by grinding instead of cutting.
Although cobalt-base alloys are not as widely used as nickel and nickel-iron alloys in high-temperature applications, cobalt-base high-temperature alloys nevertheless play an important role, by virtue of their excellent resistance to sulfidation and their strength at temperatures exceeding those at which the gamma-prime- and gamma-double-prime-precipitates in the nickel and nickel-iron alloys dissolve. Cobalt is also used as an alloying element in many nickel-base high-temperature alloys.

25th floor, C3 Building, Wanda Plaza, Kaifu District, Changsha, Hunan Province, China